Veterinary Vaccine: Process Development sub map
Once lead candidates are identified, most vaccines will undergo process development in order to create a scalable, GMP, manufacturing process. In order to reduce costs, process development is generally a non-GMP operation. This stage identifies tolerances of the manufacturing process and its robustness, reproducibility and scalability are defined and tested. This can take several months to conduct. Therefore, if possible, Early Process Development studies should be conducted in parallel with Lead product(s) identification. However, at these early stages there may be insufficient material to test, thus identification of appropriate tools for early process development remains a limitation.
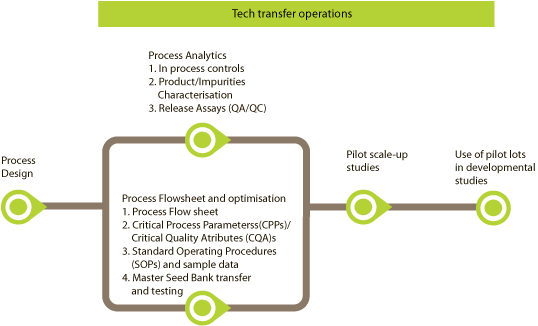
- Process Design
- Process Analytics (1. In process controls, 2. Product/Impurities Characterisation, 3. Release Assays (QA/QC) )
- Process Flowsheet and optimisation (1. Process Flow sheet, 2. Critical Process Parameters (CPP)/Critical Quality Attributes (CQA)s, 3. SOPs and sample data, 4. Master Seed Bank transfer and testing)
- Pilot scale-up studies
- Use of pilot lots in developmental studies
Tech transfer operations
As the product moves from the development phase into manufacture, it may be necessary to move site/organisation. This is most typical if the development site is a non-GMP location and small amounts of clinical material needs to be generated for Phase 1 trials, which will require a GMP manufacturer. This can be a difficult step as the equipment and scale of operation may not be matched and the two sites may not have been in communication. The step entails the transfer of materials, usually a GMP cell/virus bank, SOPs on the process and a product and process characterisation package, including key assays, that must be transferred to the GMP manufacturer. The GMP manufacturer will then have to accept the tech transfer documents and may request additional documentation/ information before they will attempt to run the process.
Normally, there are differences in equipment and scale of operation. Thus for the GMP manufacturer, they must enter a stage called Process Validation. It is during this stage that engineering batches are conducted at the desired scale of operation to see if their equipment can attain the same titres, purity and potency of the vaccine as determined in development. While material generated in engineering batches are not considered GMP material, this material can be used for animal toxicology studies if the product specification is met. It is during Process Validation that process limits can be redefined and adjusted to fit the new manufacturing site.
Once Process Validation is completed, GMP material can then be produced.
- Transfer of GMP cell banks and/or virus stocks to recipient site
- Transfer of SOPs from development site
- Transfer of analytical data package fro both product and process from development site
- Acceptance of data packages by GMP site
- Conducting engineering batches and validating the manufacturing process
- Communication between sites (both own this problem)
- Development labs have developed an unrealistic process that cannot be transferred into manufacturing.
- Tech transfer documents not fully descriptive of the process (problem owned by the development labs)
- Finding an available slot with a GMP manufacturer to make the vaccine
- Finding GMP manufacturers who have experience in the type/class of vaccine to be produced (live virus vs. recombinant)
- Novel process leads to longer process validation. In the most extreme cases the manufacturing site may need to redesign the manufacturing process for the development lab.
- Better training of development/research scientists into the manufacturing process can alleviate delays and failures at tech transfer.
- Research into the standardisation of process development and platform processes steps to de-risk scale up and tech transfer.
- Small scale GMP capacity may be an issue. Targeted investment into existing facilities may provide the most cost effective solution rather than a brand new facility.
- Process development labs also seem to be close to capacity, especially at academic centres.