Plague – Vaxonella® platform
A plague vaccine will have a dual application: primarily the development path will be in collaboration with UK and US government agencies trying to produce an anti-bioterror vaccine and then it could be expanded and developed as a vaccine against endemic disease in regions such as Madagascar. This dual nature affects the way we think about the TPP and development path.
A plague vaccine based on Prokarium’s Vaxonella platform does not have any major preclinical bottlenecks. The main bottlenecks are in the clinical phase and animal rule regulations necessary to follow for licensure. With the help of the UK Vaccine Network flow charts, Prokarium has identified four bottlenecks:
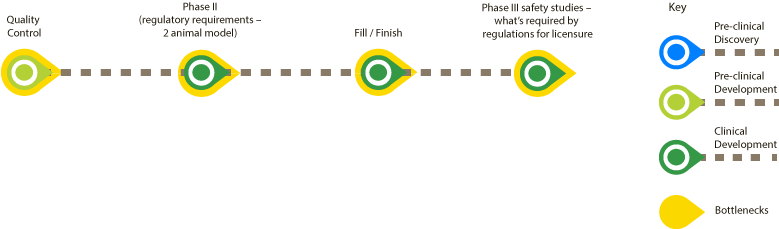
QC analytical development (for GMP manufacture) – this relates mainly to microbial safety limits and is linked to the fact that we are using a live bacterial vector.
Phase 2 by animal rule – this is very specific to plague and any diseases that are too dangerous to test in human challenge trials or for which field trials are not practical (due to low incidence). The FDA has an animal rule guide, but the EMA/MHRA does not, which for now means anyone seriously developing a plague vaccine would only do so in the US (although it should be stated that an anthrax vaccine has been licensed in the UK without human studies)
Fill-finish bottleneck (concurrent with phase 2 animal rule development and should be sorted before phase 3): there just aren't many manufacturers with fill finish capability (especially for capsules) and there is little or no new innovation or expertise in this area.
Regulatory uncertainty in regards to phase 3 requirements – this means investors will not back early clinical development, because path to licensure is uncertain.